Computational screening of N-alkylated pyridoxal derivatives is published, here you can find one of our last publications, from the Research Centre of Natural Sciences (TTK), the University of Jyväskylä and University of Turku teams.
Our manuscript entitled “N-alkylated pyridoxal derivatives as negative electrolyte materials for aqueous organic flow batteries: Computational screening” has recently been accepted for publication in the journal Chemistry—A European Journal. The main focus of the contribution is the development and analysis of a molecular database of pyridinium-frameworks.
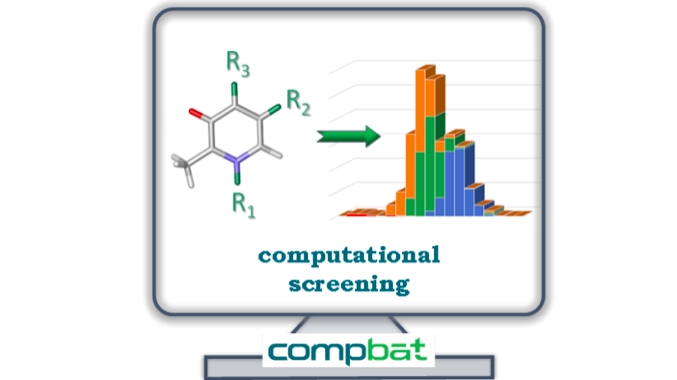
The first and highly important step towards elaborating the high-throughput screening of redox active molecules was developing a reliable workflow to generate an initial molecular database. The TTK team established a combined theoretical protocol based on different levels of quantum chemical computations which provides reliable reduction potentials. The protocol was applied to generate the structural and one-electron reduction potential of more than 6700 pyridinium-derivatives included in the B6-PYR molecular library.
The predicted reduction potentials span a broad range for the investigated pyridinium frameworks. The computational screening revealed that only pyridoxal derivatives give rise to standard reduction potentials compatible with the electrochemical stability window of aqueous electrolytes.
The ASAP version of the paper is accessible at the publisher: https://doi.org/10.1002/chem.